In a new study of water dynamics, a team of MIT chemists led by Professor Mei Hong, in collaboration with Associate Professor Adam Willard, has discovered that water in an ion channel is anisotropic, or partially aligned. The researchers' data, the first of their kind, prove the relation of water dynamics and order to the conduction of protons in an ion channel. The work also provides potential new avenues for the development of antiviral drugs or other treatments.
Members of the Hong lab conducted sophisticated nuclear magnetic resonance (NMR) experiments to prove the existence of anisotropic water in the proton channel of the influenza M virus, while members of the Willard group carried out independent all-atom molecular dynamics simulations to validate and augment the experimental data. Their study, of which Hong was the senior author, was published in Communications Biology, and was co-authored by Martin Gelenter, Venkata Mandala, and Aurelio Dregni of the Hong Lab, and Michiel Niesen and Dina Sharon of the Willard group.
Channel water and influenza virus
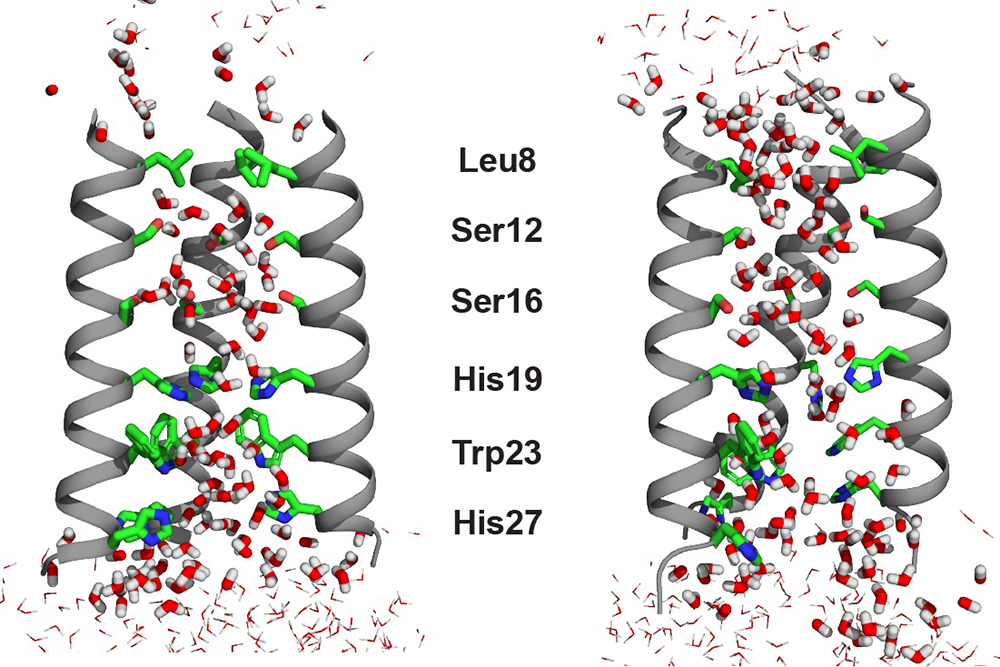
The influenza B virus protein BM2 is a protein channel that acidifies the virus, helping it to release its genetic material into infected cells. The water in this channel plays a critical role in helping the influenza virus become infectious, because it facilitates proton conduction inside the channel to cross the lipid membrane.
Previously, Hong’s lab studied how the amino acid histidine shuttles protons from water into the flu virus, but they hadn’t investigated the water molecules themselves in detail. This new study has provided the missing link in a full understanding of the mixed hydrogen-bonded chain between water and histidine inside the M2 channel. To curb the flu virus protein, the channel would have to be plugged with small molecules — i.e., antiviral drugs — so that the water pathway would be broken.
In order to align the water-water hydrogen bonds for “proton hopping,” water molecules must be at least partially oriented. However, to experimentally detect the tiny amount of residual alignment of water molecules in a channel, without freezing the sample, is extremely difficult. As a result, the majority of previous studies on the topic were conducted by computational chemists like Willard. Experimental data on this topic were typically restricted to crystal structures obtained at cryogenic temperatures. The Hong lab adopted a relaxation NMR technique that can be employed at the much balmier temperature of around 0 degrees Celsius. At this temperature, the water molecules rotated just slowly enough for the researchers to observe the mobility and residual orientation in the channel for the first time.
More space, more order
The evidence yielded by Hong’s NMR experiments indicated that the water molecules in the open state of the BM2 channel are more aligned than they are in the closed state, even though there are many more water molecules in the open state. The researchers detected this residual order by measuring a magnetic property called chemical shift anisotropy for the water protons. The higher water alignment at low pH came as a surprise.
“This was initially counterintuitive to us,” says Hong. “We know from a lot of previous NMR data that the open channel has more water molecules, so one would think that these water molecules should be more disordered and random in the wider channel. But no, the waters are actually slightly better aligned based on the relaxation NMR data.” Molecular dynamic simulations indicated that this order is induced by the key proton-selective residue, a histidine, which is positively charged at low pH.
By employing solid-state NMR spectroscopy and molecular dynamics simulations, the researchers also found that water rotated and translated across the channel more rapidly in the low-pH open state than in the high-pH closed state. These results together indicate that the water molecules undergo small-amplitude reorientations to establish the alignment that is necessary for proton hopping.
Inhibiting proton conduction, blocking the virus
By using molecular dynamics simulations performed by Willard and his group, the researchers were able to observe that the water network has fewer hydrogen-bonding bottlenecks in the open state than in the closed state. Thus, faster dynamics and higher orientational order of water molecules in the open channel establish the water network structure that is necessary for proton hopping and successful infection on the virus’ part.
When a flu virus enters a cell, it goes into a small compartment called the endosome. The endosome compartment is acidic, which triggers the protein to open its water-permeated pathway and conduct the protons into the virus. Acidic pH has a high concentration of hydrogen ions, which is what the M2 protein conducts. Without the water molecules relaying the protons, the protons will not reach the histidine, a critical amino acid residue. The histidine is the proton-selective residue, and it rotates in order to shuttle the protons carried by the water molecules. The relay chain between the water molecules and the histidine is therefore responsible for proton conduction through the M2 channel. Therefore, the findings indicated in this research could prove relevant to the development of antiviral drugs and other practical applications.
"behavior" - Google News
March 19, 2021 at 03:45AM
https://ift.tt/3cJOTqt
Chemists gain new insights into the behavior of water in an influenza virus channel - MIT News
"behavior" - Google News
https://ift.tt/2We9Kdi
Bagikan Berita Ini














0 Response to "Chemists gain new insights into the behavior of water in an influenza virus channel - MIT News"
Post a Comment